Blog
Formatting Annotated Case Report Forms (aCRFs): Part 2
This is the second in a series of posts where we answer questions from our recent webinar, Best Practices for Annotated CRFs. Below, we’ve summarized the regulatory expectations and our top insights.
Tables of Contents and Bookmarks
A table of contents with hyperlinks to bookmarked pages is seen in CDISC's sample aCRF (avaliable in the Define-XML v2.0 download). Both the Metadata Submission Guidelines (MSG) from CDISC and the PDF specifications from the FDA require aCRFs to have hyperlinks to bookmarked pages. The PDF specifications also require a table of contents.
- Dual Bookmarking
- CDISC guidance specifies bookmarks only for Visit and Form. Follow the examples of dual bookmarking in CDISC’s sample aCRF by linking multiple bookmarks to the same unique form.
- Many mistakenly think bookmarks must be in a 1:1 relationship with the form they point to. Create multiple relationships by using both of these hierarchies.
- By Visit > Visit Name > Form Name
- By Form > Form Name > Visit Name (if applicable)
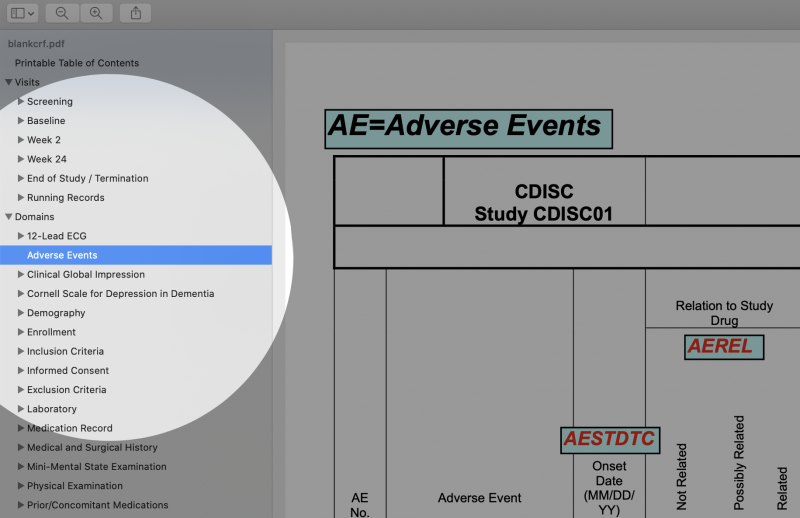
- Note: Bookmarks are not needed for Domains. Many mistakenly think the opposite, likely because CDISC’s sample aCRF has the confusing bookmark label "Domains" to mean "Forms," as seen in the image below. The MSG uses "Domains" to mean simply the topics of pages/forms.
- Note: Bookmarks are not needed for Not Submitted forms.
- Ordering and Visibility
- Forms are usually in chronological order, not alphabetical. E.g., Inclusion/Exclusion and Demographic forms are towards the beginning of the document, while End of Study forms are towards the end.
- Make a bookmark section called "Running Records" or "Log Forms" for forms that are not visit specific. These forms, such as Adverse Events, are usually at the end of the document, as seen in the image below.
- Tip: Bookmarking is often a manual task. This paper suggests how to automate it.
Document Properties
- The filename of aCRF in the tabulation package must be "acrf.pdf" per the FDA’s Technical Conformance Guide (TCG).
- Note: In Document Properties, the PDF Title and Author do not need to be populated.
- Embed fonts easily by converting to PDF/A.
- The conversion process differs depending on your PDF editing software. Try searching for "Convert to PDF/A <name of software>.
" - Note: We do not recommend flattening annotations while studies are in progress. Automation tools such as P21 Enterprise work best with non-flattened files. As a final step just prior to submission, some companies flatten files to prevent unauthorized changes.
- The conversion process differs depending on your PDF editing software. Try searching for "Convert to PDF/A <name of software>.
- The FDA requires the PDF zoom level to be "Inherit Zoom" for bookmarking aCRFs. However, when you view a file that set to "Inherit Zoom," you can still override the settings and zoom in and out.
More to Come
We hope these items give you a confident default for your workflow. For further reading, see our white paper. And please check back as we continue this series of posts!
Tags:
Blog Main Page




