January 30, 2025
For over a decade, the FDA has been using Pinnacle 21 Enterprise to ensure the accuracy of submissions. Already using Pinnacle 21 Community to validate your study date for free?


Senior Director of Product, Certara’s Pinnacle 21
Innovative leader with 20 years of clinical research and healthcare experience, specializing in acquisition, management, and transformation of clinical biospecimen and digital health assessment data. Collaborative creator of tech-enabled solutions for the pharmaceutical industry. Accomplished, analytical director possessing strong interpersonal and communication skills with experience in managing multi-functional teams at both a strategic and tactical level. Key strengths include driving transformational change, strategic planning and execution, spearheading business process improvement initiatives, and building high-performing organizations. Built and introduced countless strategies within R&D to achieve efficiencies and resolve process and application gaps. Experienced in operations oversight and guidance, including resource and financial projections and prioritization.
See how Pinnacle 21 Enterprise can revolutionize your clinical trial design process.
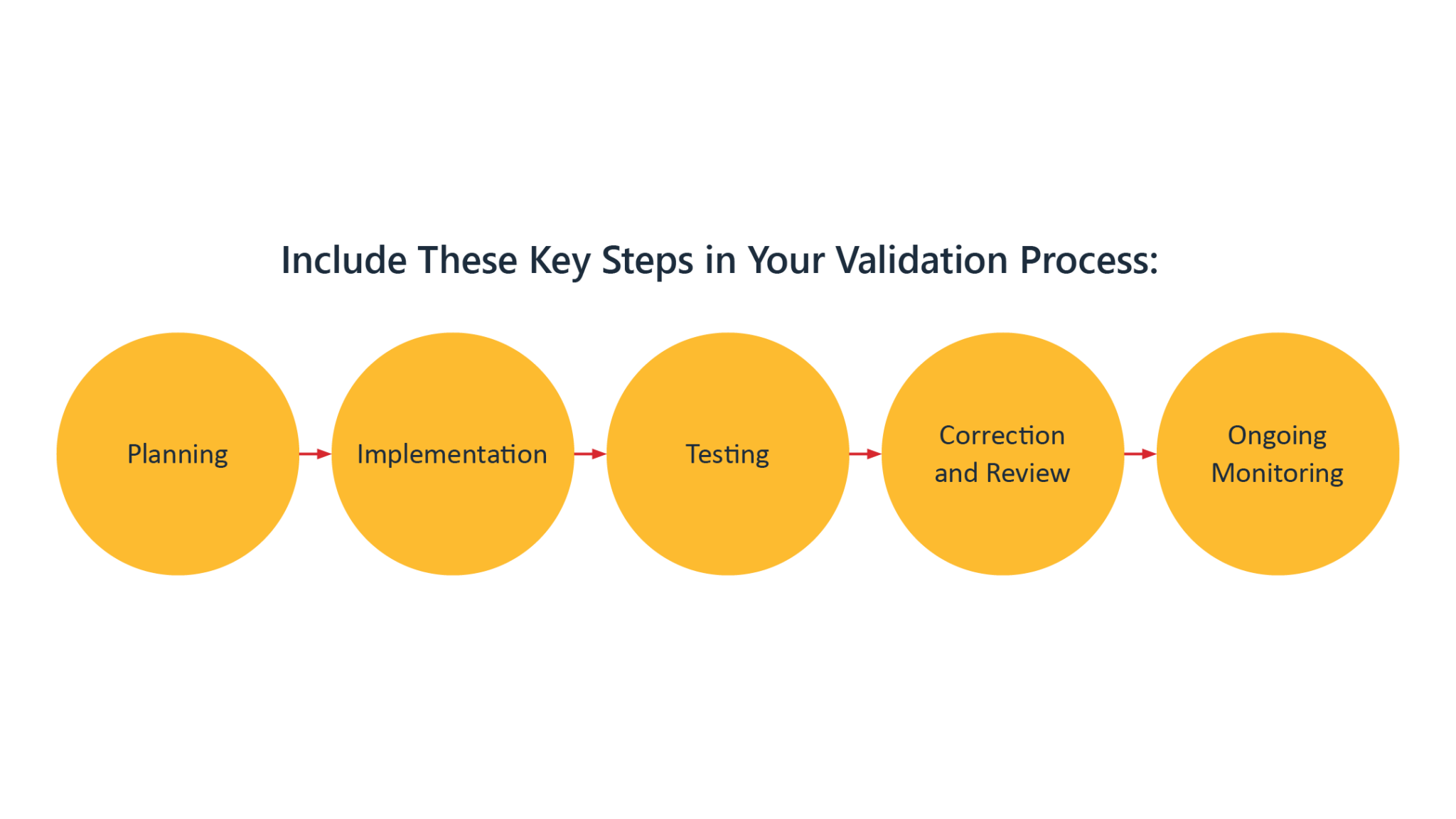
Automate compliance checks and validation.
Collaborate seamlessly in the cloud.
Gain insights and streamline regulatory submissions.