Define.xml files document the metadata that describe your tabular dataset structure. They tell reviewers the structure of your data and its origins. Many studies fail due to faulty Define.xml files.
Our Define.xml Designer is the best solution for creating these files automatically. It's intuitive. It lets you manage and edit these files directly like a spreadsheet. No need to write any code, XML, macros, or scripts. Our solution gives you one-stop support for datasets in SDTM, SEND, ADaM, and Analysis Result Metadata.
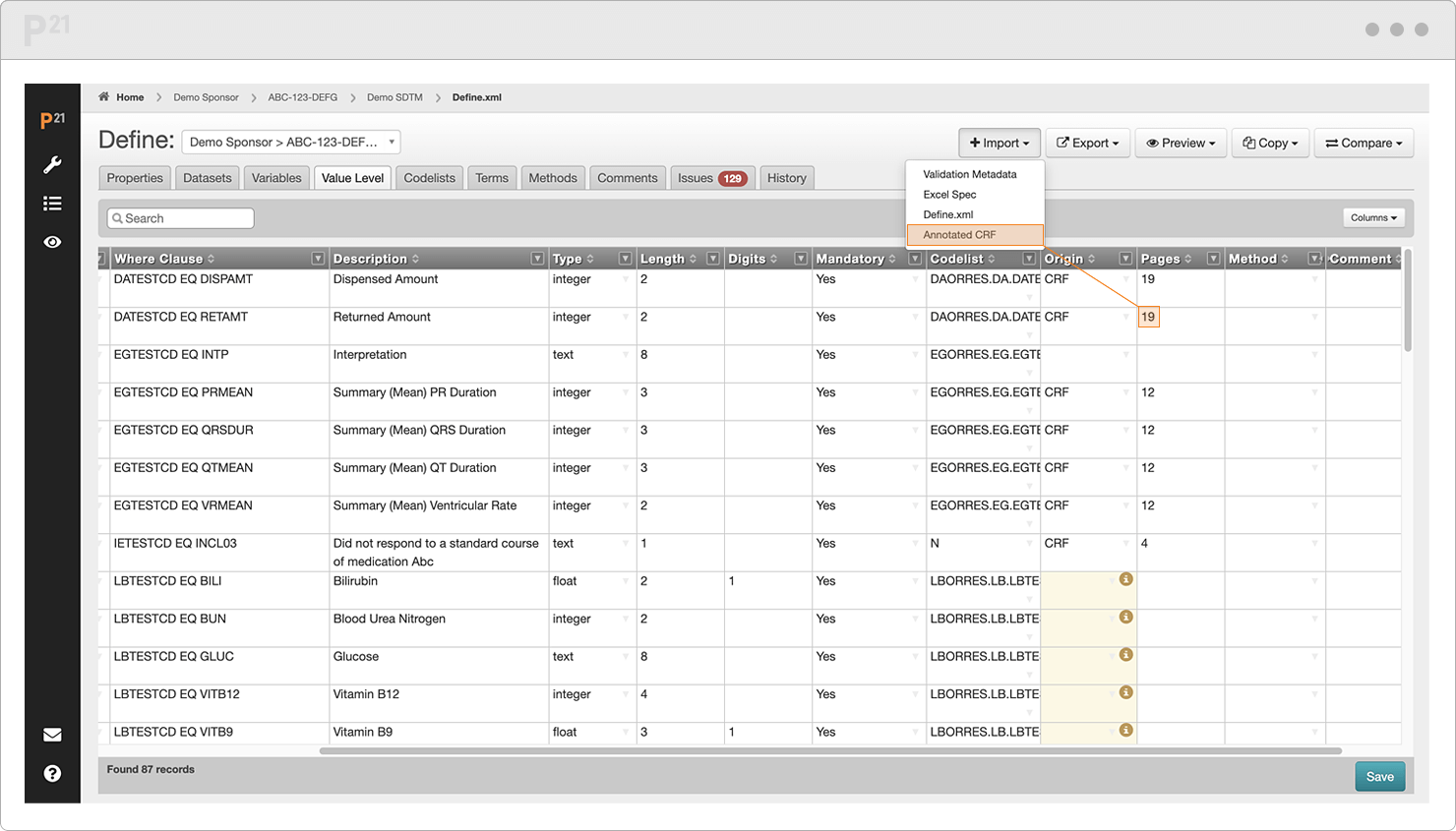
Extract dataset metadata for domain, variable, codelist, and value level. Then merge with standards.
Autofill annotated CRF page numbers. (Clinical data teams smash the like button for this.)
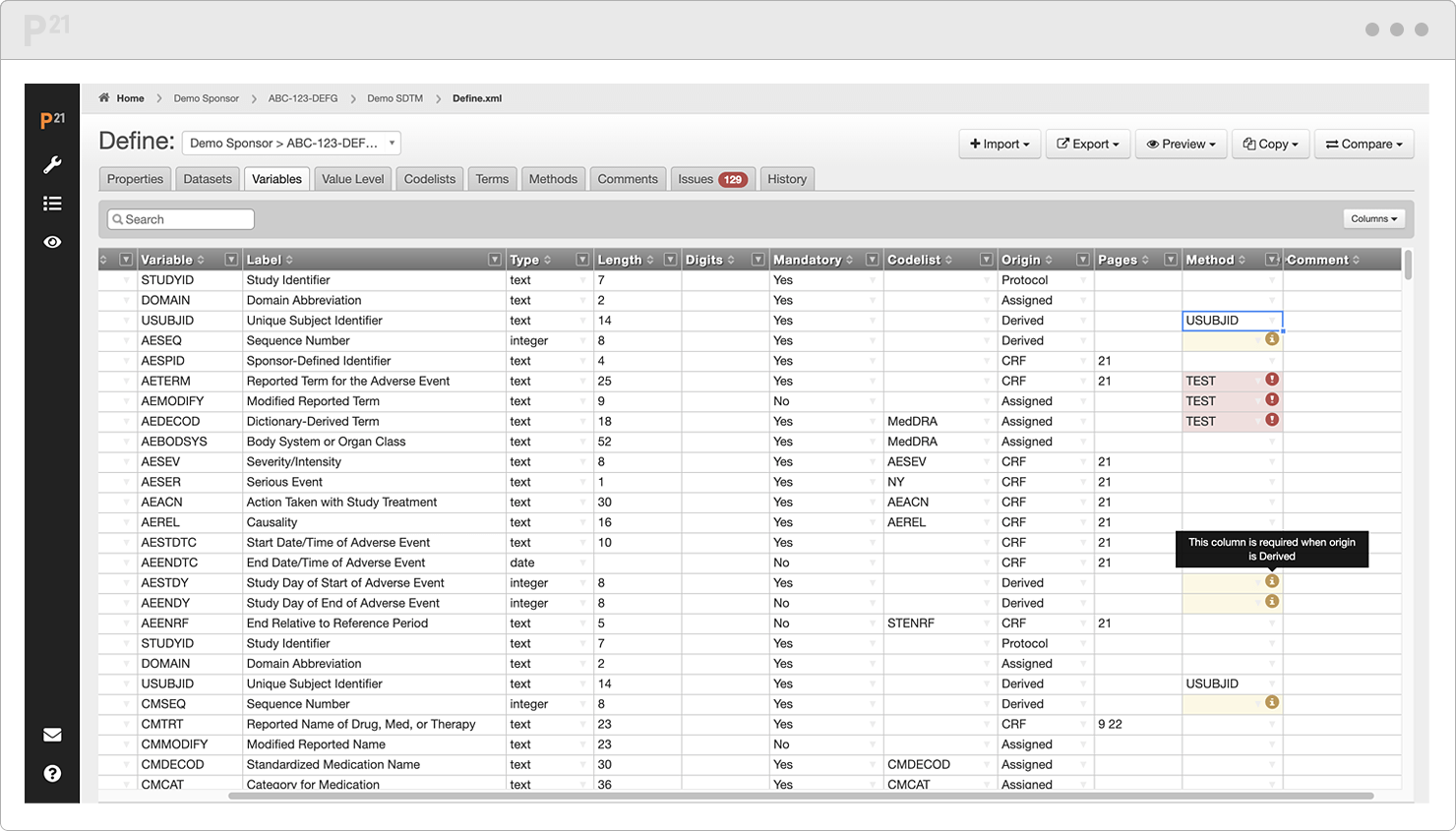
Spot errors and invalid entries as they are highlighted in real time.
Import spreadsheets for instant issue flagging and auto-conversion to XML format.
Update and version your Define.xml files with total control. Metadata management made easy.
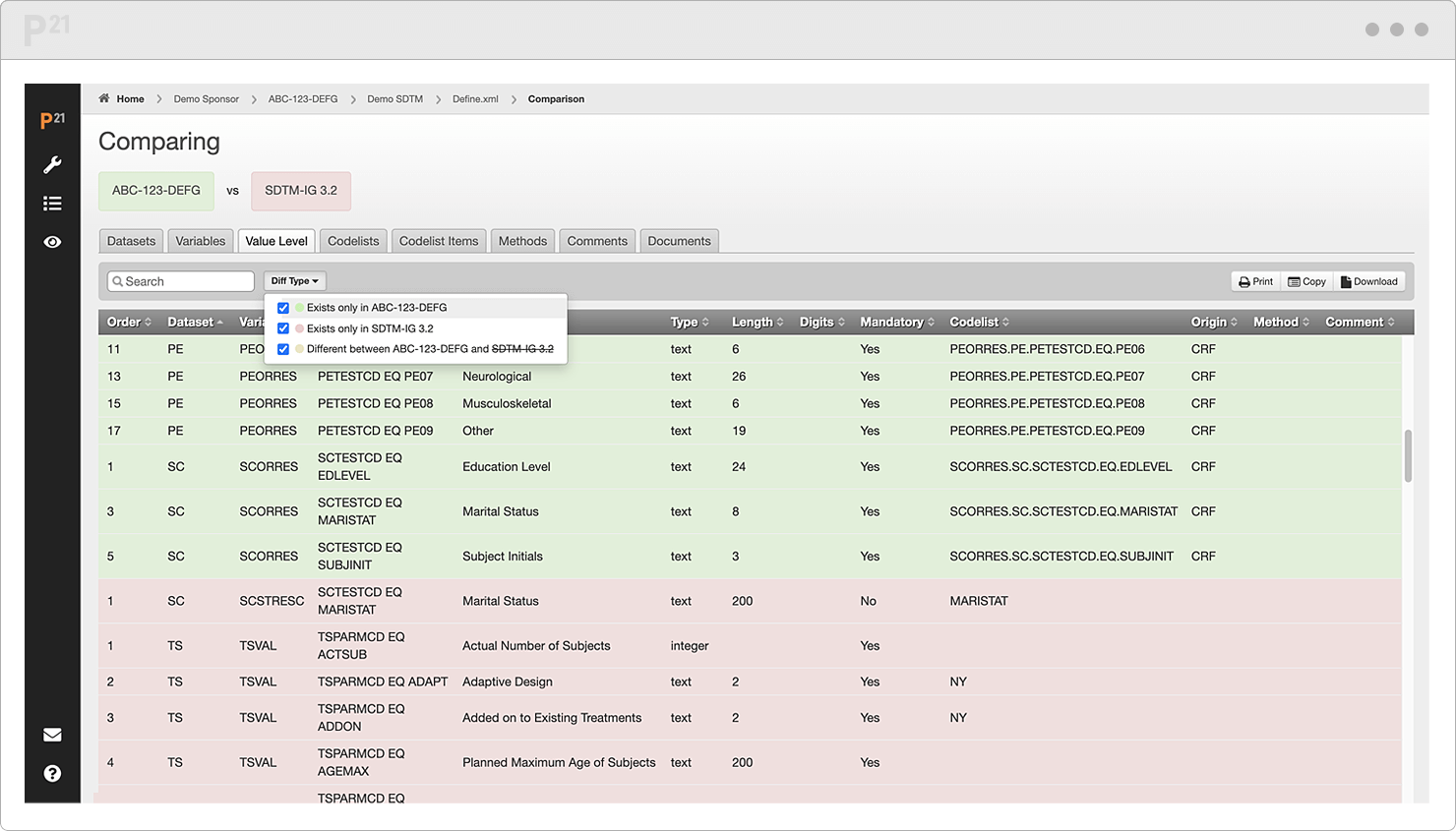
Compare studies—or versions of a study—to visualize their deltas over time.