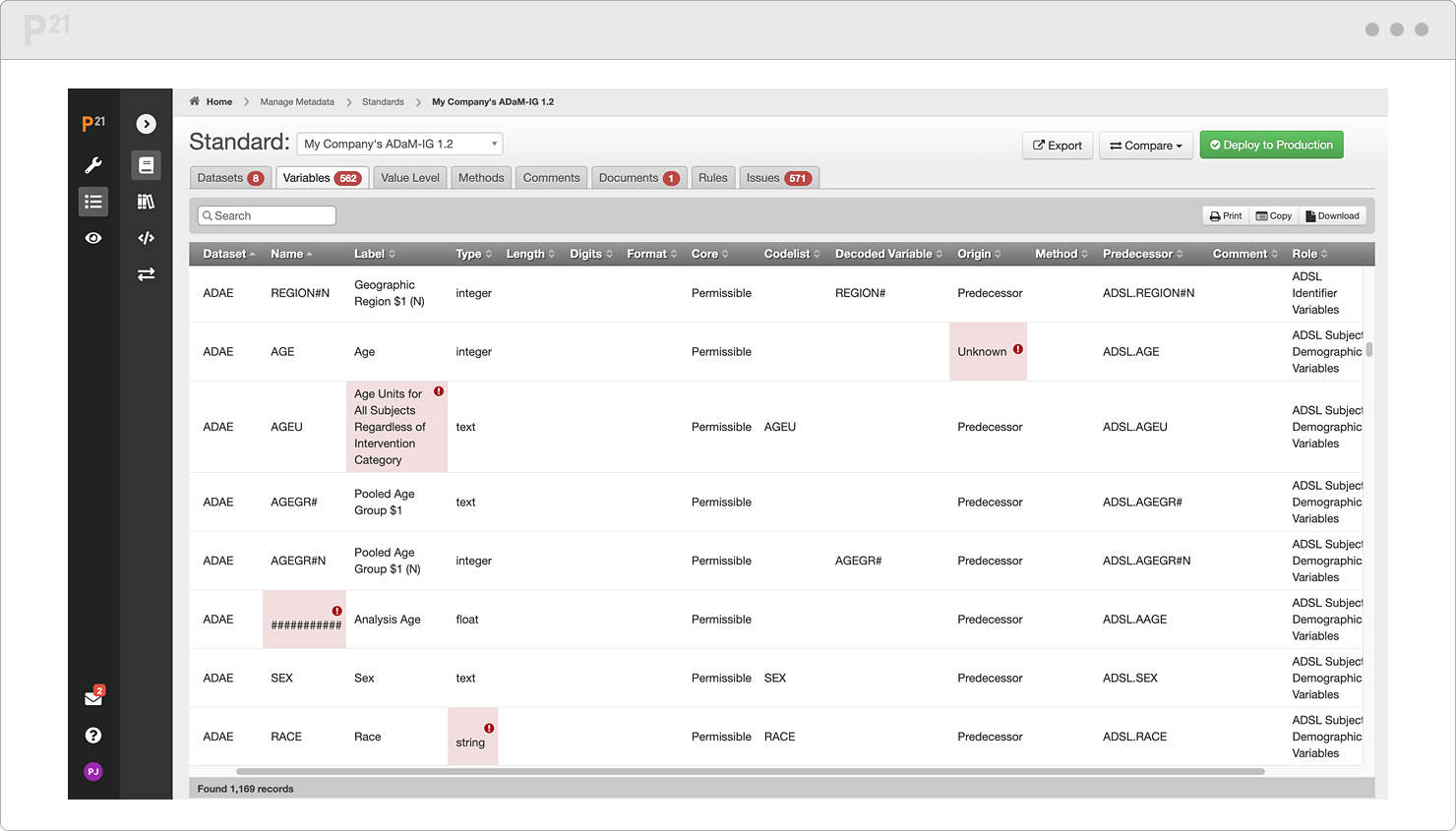
Our validator makes data standards more actionable and less "up for interpretation." It's the same tool used by the FDA and Japan's PMDA to review the quality of submissions. Judge datasets against their rejection criteria to fix issues in advance. With all on the same page, you mitigate risk and accelerate the review process.
Use the dashboard and scoring algorithm to monitor progress toward submission readiness. Track changes between validation runs with clear before and after views. And dig deeper into your data composition with our suite of over twenty pre-built reports. Even without a CS degree, you can surface patterns and inconsistencies.
The final checkpoint before datasets embark for the FDA, PMDA, or China's NMPA. It checks whether data comply with CDISC standards, controlled terminology, and dictionaries like MedDRA and WHODrug.
It also checks whether data follow each agency's business rules. Issues may derail their standardized review tools. So we offer insights and "Fix Tips" to stay on track.
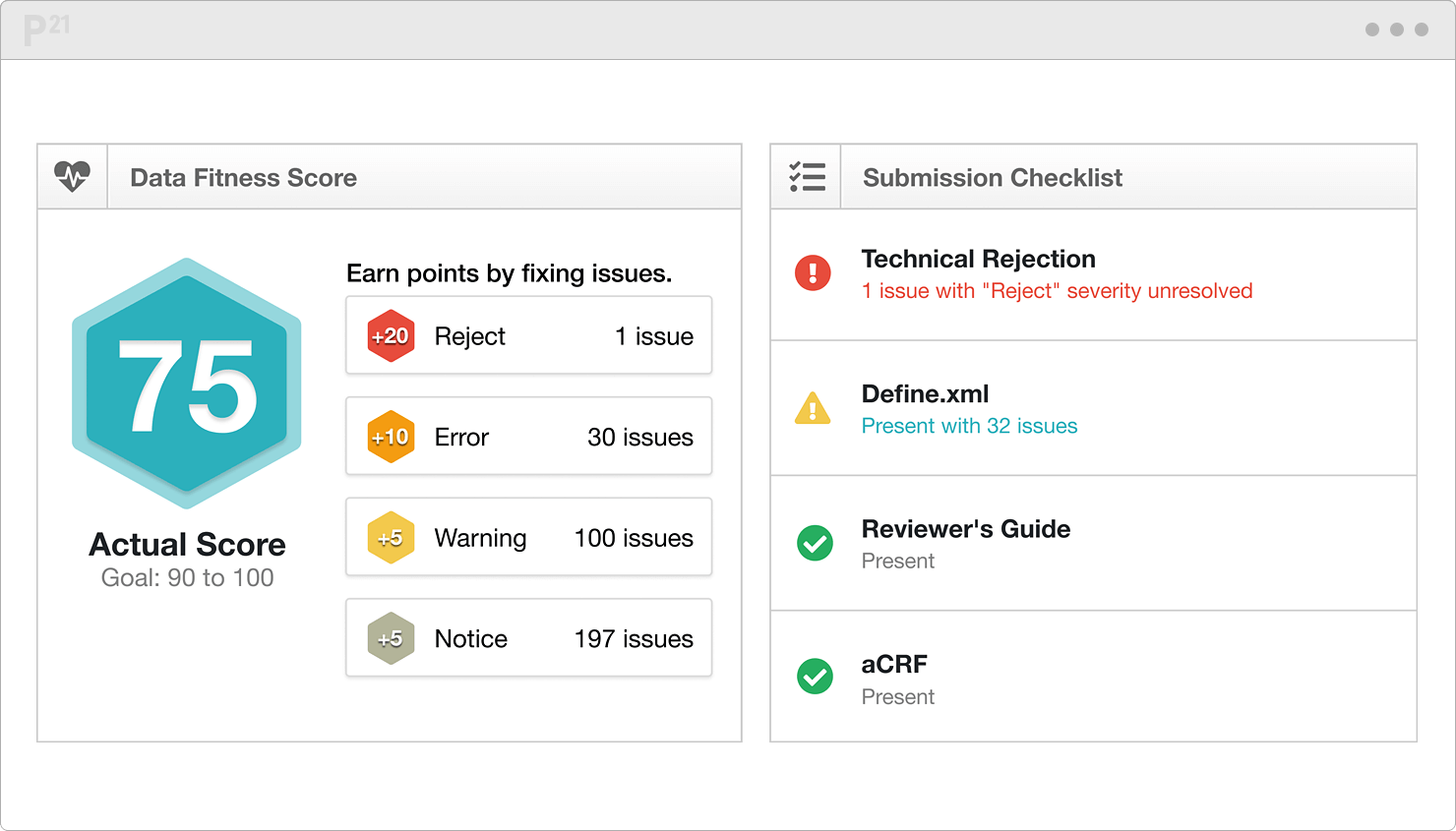
Fix quality issues at data cuts while studies are ongoing. Validate data early and often. The feedback loop helps both you and your collaborators.
Sponsors can share access to the platform with partners and vendors. Ensure their deliverables are perpetually compliant and submission-ready. This isn't a test to cram for.
Does your company create its own internal standards? Upload your custom standards, terminology, and business rules into our validator to see which are CDISC-compliant and which need work.
Our platform is essential in guiding partners and CROs. Ensure data are validated your way, and enforce your specifications.