Blog
FDA’s New Validation Rules Explained
FDA Validator Rules Version 1.6 Explained
In December 2022, the Food and Drug Administration (FDA) published an updated version of its Validator Rules. Pinnacle 21 Principal Consultant Michael Beers delivered an overview of the differences between newly released version 1.6 and the now legacy March 2021 version 1.5.
Note: Michael Beers is a Pinnacle 21 staff member and in no way speaks for the regulatory agencies or standards development organizations. His presentation was delivered on behalf of Pinnacle 21.
What is Validation and where are the New FDA Validator Rules?
We begin with Section 8 of the FDA Study Data Technical Conformance Guide, where data validation is defined as a way to help ensure that the study data are compliant, useful, and will support meaningful review and analysis at the agency.
At the agency, validation occurs at the time of submission and at the beginning of regulatory review, where submitted data is checked against various rules and standards, including:
- Standards provided by organizations like CDISC, which check that data conforms to published standards. When CDISC publishes new versions of the standards, in most cases they provide CDISC Conformance Rules that specify exactly how the data should be checked for conformance to that new version of the standard.
- FDA eCTD Technical Rejection Criteria, which specify the validation criteria applied during processing of new submissions. These change infrequently.
- FDA Business and Validator Rules, which describe the business requirements for regulatory review. FDA validator rules are the actual detailed checks that make sure the business requirements are met.
So, where can you find the FDA Validator Rules? Navigate to the FDA website to the Study Data Standards Resources page. When expanding the section titled FDA Validator and Business Rules, you’ll find a link to download the rules.
What changed in the FDA Validator Rules from v 1.5 to v1.6?
3 New Versions of CDISC Standards and 118 New Validator Rules
Three new columns have been added for the addition of SENDIG 3.1.1, SENDIG-AR 1.0, and SENDIG-DART 1.1 to the FDA Data Standards Catalog. This is one of the biggest changes to the Validator Rules in version 1.6.
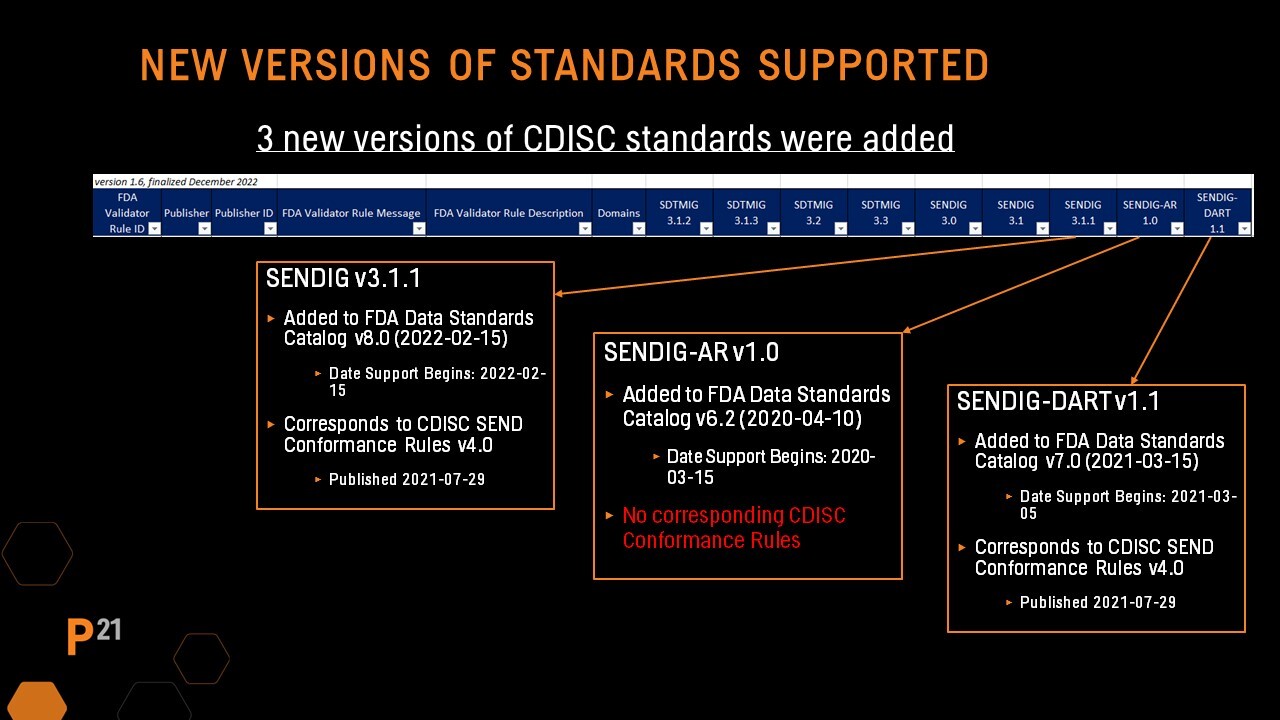
- SENDIG 3.1.1 was added to the FDA's Data Standards catalog in February of 2022. The rules that coincide with this standard are from CDISC SEND Conformance Rules 4.0, which was published in July of 2021. This update includes the addition of 4 new rules.
- SENDIG-AR 1.0 was actually added back in 2020. For this standard, there are no corresponding CDISC Conformance Rules. This includes 61 new rules, most of which have no Publisher ID because there are no corresponding CDISC Conformance Rules.
- SENDIG-DART 1.1 made its way into the FDA Data Standards Catalog in March of 2021. This standard corresponds to CDISC SEND Conformance Rules 4.0. You’ll find 20 new rules added here.
The other 33 rule additions were to meet a specific need; for example, medical device rules. Five of these are new SDTM rules, and another five are applicable to both SDTM and SEND. The remaining 23 new rules are for SEND only.
Updated Publisher IDs
Note that three of the new SDTM rules have no publisher ID because there are no corresponding CDISC Conformance Rules for medical devices, and four of the SEND rules have no publisher ID because there's no corresponding CDISC Conformance Rule or FDA Business Rule.
Publisher IDs were updated for 11 rules where CDISC Conformance Rule IDs were added to existing rules. These changes affect SEND Conformance Rules 4.0 (2), SDTMIG Conformance Rules 2.0 (8), and 1 missed CDISC Conformance Rule ID that has now been corrected.
Rule Message and Rule Description Updates
Three rules received message updates, and rule descriptions were updated for 14 more. These changes were largely housekeeping to correct typos and reformat to achieve more consistency with other rule messages and descriptions.
Changes to SDTMIG Rule Assignments
- Added to all SDTM versions: SD1138 (--DRVFL=’Y’, when –ORRES value is populated). This appears to have been mistakenly unassigned previously.
- Removed from SDTMIG 3.1.2, 3.1.3, and 3.2: SD1259 (Invalid value for SETCD). This SETCD variable only exists in SDTMIG 3.3 and is not applicable to previous versions.
- Removed from SDTMIG 3.3: SD0063 and SD0063A (SDTM/dataset variable label mismatch). The reason here is matching the variable labels is no longer part of conformance in this version of the standard.
Changes to SENDIG Rule Assignments
- Added to SENDIG 3.1: SD1118 (Neither –STDTC, --DTC nor --STDY are populated). The reason again is that it appears to have been mistakenly unassigned.
- Removed from SENDIG 3.1: SD1008 (CODTC is populated, when comment is a child record of another domain). The corresponding CDISC Conformance Rule does not apply to SENDIG 3.1 or later.
- Removed from SENDIG 3.1: SD1138 and SD0063A (--DRVFL=’Y’, when --ORRES value is populated). The reason here is the original result can be populated and send findings to means when the dried flag equal to yes. This is different from SDTM.
How to stay compliant with new Validator Rules
If you're an Enterprise user, we recommend that you always accept the latest Enterprise deployments and that you always use the latest available regulatory engines. For ongoing, in-progress studies in Enterprise, a best practice is to use the latest P21 engine, which will show you the issues for FDA, PMDA, and rules for custom standards and controlled terminology (if your company uses these). Later, when a study is finalized and approaching submission, switch to the most recent agency engine.
According to Michael Beers, when preparing to submit, “you always want to make sure that you're using the latest agency engine for FDA. You do not want to use the legacy FDA engine. This is provided just for reference.”
When you switch to the end agency-specific validation engine, such as FDA 2204.1, you’ll still see the same FDA validation issues, but you will only see the FDA issues.
If you were to use a legacy FDA engine instead of the most recent version, you would see warning messages on various screens, such as in the Data Package Details, the Submission Checklist, and even in the Validation Report. Before exporting from Enterprise, you would also see red warning messages directing you to revalidate using the latest engine.
Failure to validate with the latest engine means you won't see the exact same validation results as the agency, so don't ignore those warning messages!
If you're a Community user, the same best practice applies here. Always be sure you are using the latest version of Community and connected to the internet, and select the latest FDA engine, never the legacy engines provided strictly for reference. If you were to use an outdated engine, you would receive an “incompatible engine” warning message in the validation report.
What to do with validation issues once you validate your data:
In section 8.2.2 of the FDA Study Data Technical Conformance Guide, the FDA recommends that all Sponsors evaluate their study data before submission against the conformance rules. Here at Pinnacle 21, our mantra is that you should validate as soon – and as often – as you can.
The sooner you validate, the more likely you are to be able to fix any validation issues identified. The longer you wait to validate, fixing issues becomes more difficult due to impact on downstream deliverables.
Once you’ve validated, try to fix as many of those issues as you can. According to the FDA, “Sponsors should either correct any discrepancies between study data and the standard or the business rules or explain meaningful discrepancies in the relevant Reviewer’s Guide.”
Questions and Answers
Q. Do we need to switch to the newest validation engine if we started on a previous validation engine for an ongoing study?
A: Yes, the recommendation is always to switch to the latest agency validation engine. We understand that you may see differences from a new validation engine, but this is only good. Surfacing new issues prevents you from being surprised by FDA reviewers who have the most up-to-date version of the Validation Rules. If your team is facing time or budget constraints, you can always add an Issue Explanation to such newly flagged Issues.
It's always good to explain every issue that the agency will see in your Reviewer's Guide, even if you're unable to fix it. Sometimes the explanation is simply that the engine came out too late for you to fix, but it's best to know in advance what issues the agency will see so you know you have explanations for each.
Q. How often does the FDA plan to update their Validator Rules?
A. It appears that the goal is to publish rule updates each year. Version 1.5 was released about 18 months ago, so that can fluctuate a bit.
Q: Why isn't severity included in the FDA Validator Rules anymore?
A. This is not new to version 1.6. It was changed in a previous version of the Validator Rules. CDISC does not provide any severity for their Conformance Rules, and the FDA business rules don't have any severity either. FDA Validator Rules do not publish severity.
We have kept severity for our Enterprise clients, but we’ve changed it to “Type.” There are three values associated with Type - error, warning, and notice. Many organizations use these values as part of their validation issue management process, so we’ve maintained it for our Enterprise users.
If we missed any burning questions or topics, check out the full webinar and slide deck to see if we covered it. If you still have other questions, please feel free to contact us!
Tags:
Blog Main Page




