Blog
Pinnacle 21 Releases P21 Community 4.0
As of April 25, 2022, we have released P21 Community 4.0 to general availability! Install it now from our Downloads page.
P21C 4.0 is the first major release of this application since P21's M&A with Certara in October 2021, per the commitment that P21's software applications will “continue to be supported, updated, and developed.”
We've invested heavily in P21C 4.0, with functional changes across many of its features and support for key standards, and some quality-of-life bug fixes too.
Release Highlights: Application
Our previous major P21C release was P21C 3.0 in May 2019, the release that introduced the concept of decoupled, agency-specific Validation Engines. Regulatory agencies and our industry users have advanced a long way since then. Below are the main features and updates waiting for you in P21C 4.0.
Support for the Define-XML 2.1 Standard
P21 strongly supports the adoption of this new standard for Define-XML, as it introduces combinatorial standards/versions, Class vs. Subclass, Origin, and handling for No Data. (In fact, Define-XML 2.1 support, in addition to rich GUI-based metadata editing features, have already been available to P21 Enterprise clients as early as 2021.)
- In the Define.xml Generator under Create Spec, the P21 Excel template now follows the new format. Also, the old template will still work, as we’ve maintained backwards compatibility.
- Similarly, under Generate Define, you now have a drop-down for Define Standard from which you can select Define-XML 2.1. Via CLI, you can specify this with a new parameter (--source.define.standard).
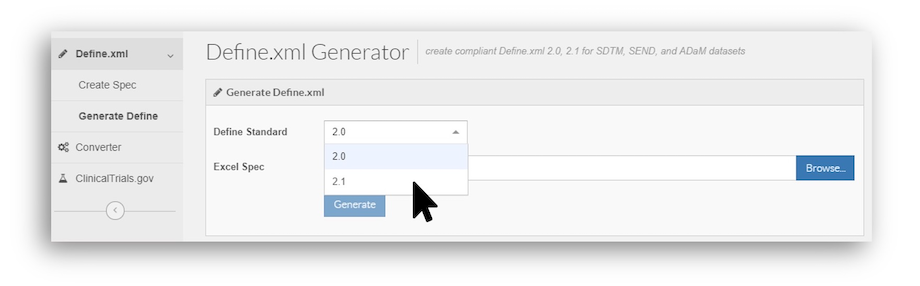
Per the FDA’s 2022-02-16 Data Standards Catalog (DSC), Define-XML 2.1 is supported from 2021-03-15, and required from 2023-03-15, 2 years after support begins. Note: These dates are based on study start date, not submission date. Refer to the FDA DSC or consult with your reviewers for details. Many studies may continue to use Define-XML 2.0, as its support has not retired at the FDA yet.
Support for Analysis Results Metadata
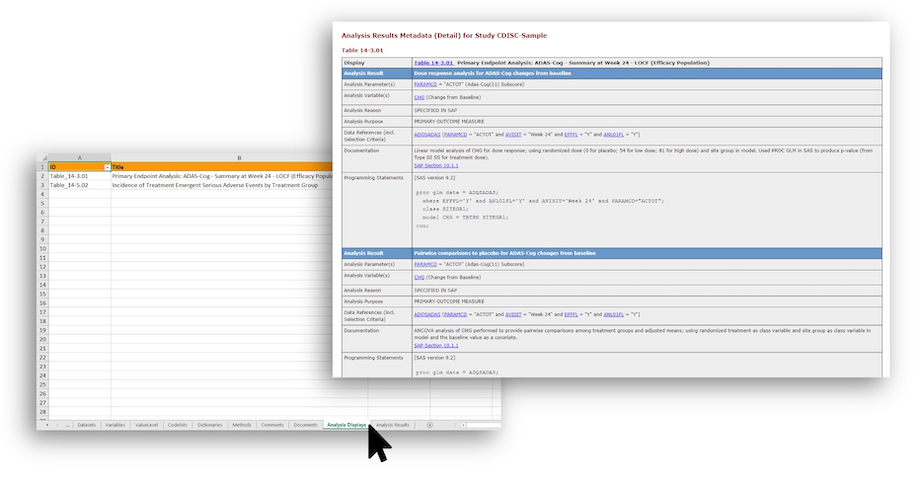
Analysis Results Metadata (ARM) has now come to P21 Community! For Users working with ARM for ADaM datasets, ARM manifests as separate worksheets in your define.xml.
- ARM provides traceability from results in a statistical display to the data in the analysis datasets. It helps regulatory reviewers understand and reproduce analysis results. ARM is an optional ADaM metadata component according to ADaM v2.1. For example, ARM may be provided for complex statistics (e.g., p-values) but not necessarily for descriptive statistics like mean or median. However, best practice is to provide it to assist the reviewer in their assessment of the critical analyses.
- ARM has two parts: Analysis Displays and Analysis Results. A display may include one or more analysis result(s). The metadata for an individual result 1) provide links between the result, external documentation, source ADaM dataset(s), and 2) document the analyses performed, either textual in the documentation section or with the aid of programming code in the programming statements section.
Support for the LOINC Dictionary
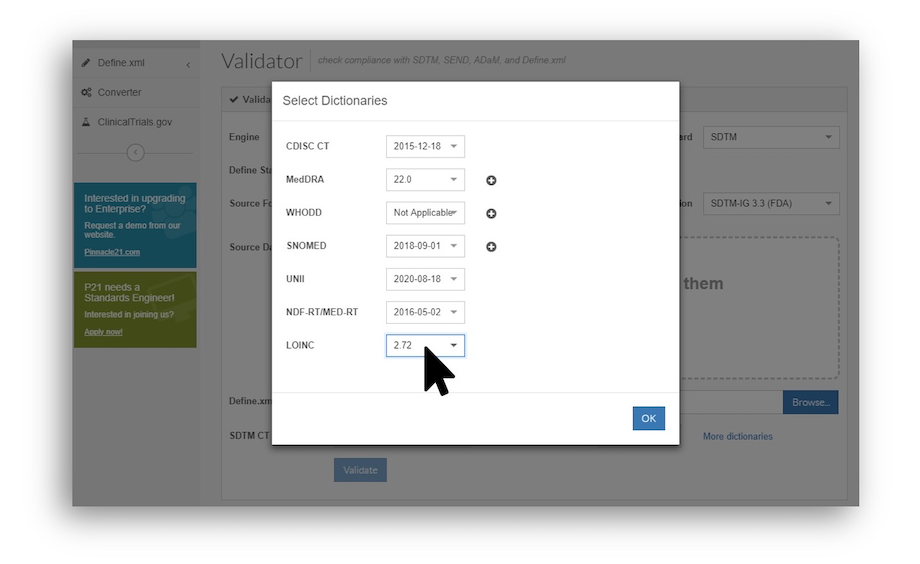
Beginning with P21C 4.0, LOINC support is now embedded in the application.
- At first, you will see only one LOINC version available in the Validator. But going forward, as additional LOINC versions are released, P21C will automatically synchronize and add additional options to the LOINC dropdown. (For those looking for the backwards-facing versions of LOINC too, P21 Enterprise contains the superset of past and present.)
- Note: LOINC is valid for SDTM validations only, and should be included only if provided in the data by the actual labs doing the collection. Via CLI, you can specify this with a new parameter (--loinc.version).
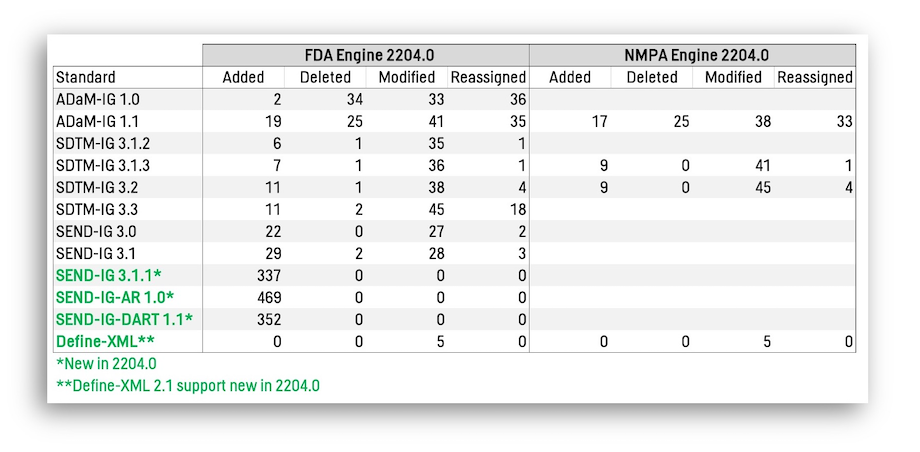
Release Highlights: Validation Engines
In addition to our first-in-industry support for CDISC CORE via the new CDISC 0000.1 Engine, the table below summarizes all changes in the Rules for the regulatory Engines for the FDA and the NMPA.
- We now support multiple new standards (green): SENDIG 3.1.1, SENDIG-AR 1.0, SENDIG-DART 1.1, and Define-XML 2.1. Users doing nonclinical work especially benefit from this upgrade.
- The Added Rules have the most impact, with the Modified Rules having lesser impact, as Modified may include both major updates to algorithms and minor updates to Messages, Descriptions, etc.
- Note: For P21 Enterprise clients, there has already been a release of the Enterprise-only P21 Engine 2204.0, with 1,400+ added Rules, exclusive support for SDTM-IG 3.4, ADaM-IG 1.2, and FDA’s BIMO 2.0. Contact your Customer Success Manager for additional information.
As you explore these new updates, we are always eager to meet you at conferences such as PHUSE and PharmaSUG and support you via the P21 Community forum to advise on best practices, technical details, etc. Also, check your email inbox later for an invite to our upcoming educational webinar on P21 Community 4.0.
Tags:
Blog Main Page




