Blog

OpenCDISC Live is making its way to North Jersey for the second stop on
our 2014 tour!
Our first OpenCDISC Live event — in Boston, earlier this summer — drew more than 100 attendees, and a wealth of thoughtful discussions around JumpStart, DataFit, and the modern FDA approval process.
The “Creating Define.xml 2.0 with OpenCDISC” webinars that ran on August 12th and 14th seemed to have served a big need. We had a total of 311 attendees across the two webinars, and received many thoughtful questions.
Bottom line: a lot of you have been struggling with Define.xml, and are excited to have a tool that can create FDA-compliant Define.xml 2.0 files so quickly and assuredly. But, as with any new technological solution, there is a learning process involved.
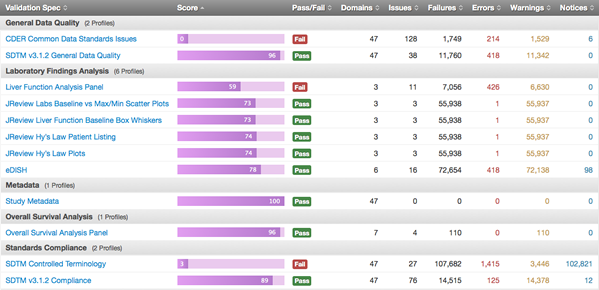
There has been a lot of buzz about DataFit of late. The FDA announced it as a new project that will enable the organization to “effectively leverage standard data to advance the review process.” But this new project is more than ten years in the making.
OpenCDISC Live is making its way to North Jersey for the second stop on our 2014 tour! Enjoy this opportunity
to meet the developers of OpenCDISC face-to-face, ask questions, collaborate and learn.
In our second Forum, we'll be discussing how to prepare for the new FDA JumpStart and DataFit processes. We'll also give you an in-depth look at OpenCDISC Enterprise and show you how its new advancements can assist the process.
Registration is open for the next meeting, scheduled for September 17, 2014 from 1-5pm. The meeting will be hosted by by Bristol-Myers Squibb at their Hopewell Campus. Web conferencing will also be available. Please register by Friday, September 5. The link to register: https://www.survey-expert.com/surveyIndex.asp?U=6009005008101025352
Meeting agenda:
- Alex Loboda, Eisai: How to ensure good quality data in your submission
- Vineet Jain, independent consultant: Robust approach to create Define.xml 2.0
- Marcelina Hungria, DIcore Group: SDTM / Overview and Status of the new Ophthalmic Examinations (OE) Domain Mode
- Vincent Guo, Novartis: Model X-Ray Image Data into ADaM BDS Structure – Specialty Data for a Complex and Important Efficacy Endpoint that Assesses Joint/Bone Structural Damage and Progression
- New feature: Panel discussion
See you there!
You’ve probably heard of JumpStart by now. The FDA is describing it as a “service [that] is modernizing the drug review process.” But how, and why? And how can the pharmaceutical and biotech industries benefit from it?
One of the big drivers of this change was time. Currently, when the FDA receives a submission, reviewers spend up to 45 days just to assess the quality of that submission. They have to first churn through enormous files — some of which are gigabytes in size — to determine if the data quality and content are strong enough to support the review. Between the time spent by the FDA to conduct this “pre-review,” and the time spent by the submitting organization to fix these issues and resubmit, valuable months are often wasted.

Thanks to all who attended the first OpenCDISC Live Forum in Boston, MA!
This inaugural CDISC and FDA educational event was a resounding success. Topics covered included the challenges and opportunities presented by JumpStart, DataFit, and the modern FDA approval process.
 Pinnacle 21 proudly introduces OpenCDISC Live!
Pinnacle 21 proudly introduces OpenCDISC Live!
This series of events will travel across the U.S., throughout 2014 and beyond. They will provide time and space for pharmaceutical and biotech professionals to meet with the developers of OpenCDISC, learn what’s coming from the FDA and CDISC, ask questions, collaborate and learn.
Welcome to the first ever OpenCDISC Live Forum - a series of events where you can meet the developers of
OpenCDISC face-to-face, ask questions, collaborate and learn.
In our first Forum, we'll be discussing how to prepare for the new FDA JumpStart and DataFit processes.
We'll also give you an in-depth look at OpenCDISC Validator 1.5 and show you how it's new advancements
can assist the process.
We're pleased to announce the availability of OpenCDISC Validator 1.5.
Download OpenCDISC Validator 1.5 here
What’s New in Version 1.5
- Validation against SDTM 1.4 model and SDTMIG v3.2
- Validation of Define-XML v2.0
- Support for Dataset-XML format
- Updated SEND config for FDA compliance
- Various bug fixes and minor engine enhancements. Please refer to the changelog for details.
- Various updates to SDTM 3.1.3, 3.1.2, 3.1.1, and SEND 3.0 configs. For a complete list of validation rule changes please refer to the release notes.





